
We can establish the relationship between the atomic mass unit and gram with the help of the Avogadro constant (N A). In chemical laboratories, chemists prefer more quantifiable units like g mol 1. It is defined as 112 of the mass of an unbound neutral atom. It is defined as one-twelfth (1/12) of the mass of an unbonded Carbon-12.This is equal to 1.66053892 × 10-27 kilograms, making it useful for talking about the mass of individual atoms or molecules. The atomic mass unit or the unified mass unit is a non-SI unit, and it is not a practical unit of mass. The dalton or unified atomic mass unit is a unit of mass widely used in physics and chemistry. This is because an atom's mass is affected very slightly by the interactions of the various particles within the nucleus, and the small mass of the electron is taken into account. An atomic mass unit, or AMU, is a physical constant in chemistry equal to one-twelfth of the mass of an unbound carbon-12 atom. The atomic mass unit (u or amu) is one unit for measuring the atomic mass of particles. Name chemical element: Symbol: Atomic number - Name alphabetically: 1.
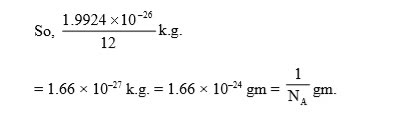
The chemical elements of the periodic chart sorted by: Atomic Mass. This list contains the 118 elements of chemistry. The atomic mass (or the atomic weight) of an element may now be defined as the average relative mass (or weight) of its atom compared with one atom of carbon taken as 12. click on any elements name for further information on chemical properties, environmental data or health effects. This unit called the atomic mass unit (a.m.u) is defined as 1/12 the mass of one 12C atom. An atomic mass unit is a unit of mass used to express atomic and molecular weights, equal to one twelfth of the mass of an atom of carbon-12. However, as seen by the helium and sulfur examples, the masses of individual atoms are not whole numbers. The elements of the periodic table sorted by atomic mass.


 0 kommentar(er)
0 kommentar(er)
